|
|
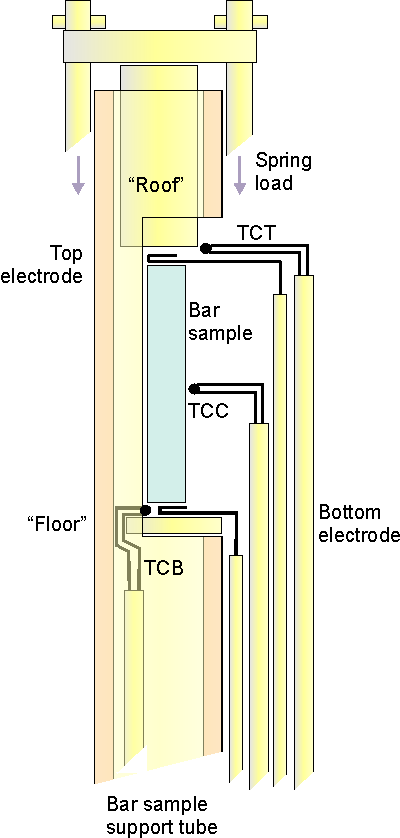
The ProboStat can be used to measure Seebeck coefficient in many ways. The standard method is to suspend a bar sample between two thermocouple tips and measure temperatures at both ends and then the sample voltage between the + or - leads of the thermocouples, or use separate electrodes for sample voltage measurement as in the picture. Temperature gradient over the sample is either naturally occurring furnace gradient or one induced by small internal heater placed above the spring load top plate. This method can also be used in combination with 4-point conductivity measurement. This method can be be done from ambient temperature up to 1600ºC, from vacuum to high pressure (high pressure option), with any type of gas atmosphere. Best results are achieved with bar sample of 3-4 cm length. Sample may be of arbitary shape but it is easier to mount if the sample is bar shaped with longer length than cross section.Disc and thin film samples are not mounted as easily as bars and the electrode contacts may have to be glued (with Pt ink) to the side (since the film only covers the side and not the ends of the sample). There is no clear limit how small samples can be measured, but as the sizes go smaller the mounting gets harder and more importantly the ratio between signal and error gets less favourable. This method involves reading two thermocouple voltages and the sample voltage, converting the thermocouple voltages to temperatures, calculating the temperature difference and plotting the sample voltage against the temperature difference. This can be performaed by the user or automatically with the Omega software. NORECS can provide with full system (including ProboStat sample holder, furnace, Omega software, multimeter and power supply for the internal heater) that can automatically perform all the tasks needed for determining Seebeck coefficient at various temperatures. ProboStat and Omega offer well proven and repeatable way of measuring Seebeck coefficients but also allows user great freedom to alter the setup to match for the specific qualities and challenges each individual sample has. This freedom and control over every aspect is the key to reach accurate result164 and is missing in 'turn-key' systems. It is possible to combine this method with four point resistance measurement by adding two additional electrodes on the sample, see combined Seebeck coefficient and conductivity measurement Seebsys - Combined Seebeck coefficient and resistance measurement system |
These articles refer to ProboStat or other NORECS products, filtered with keywords: 'Seebeck, Thermoelectricity, Thermoelectric '

Experimental application of a laser-based manufacturingprocess to develop a free customizable, scalablethermoelectric generator demonstrated on a hot shaft
| Authors |
|
| Source |
Engineering Reports
Time of Publication: 2022
|
| Abstract | Geometry, design, and processing in addition to the thermoelectric materialproperties have a significant influence on the economic efficiency and perfor-manceofthermoelectricgenerators(TEGs).WhileconventionalBULKTEGsareelaborate to manufacture and allow only limited variations in geometry, printedTEGs are often restricted in their application and processing temperature due totheuseoforganicmaterials.Inthiswork,aproof-of-conceptforfabricatingmod-ular, customizable, and temperature-stable TEGs is demonstrated by applyingan alternative laser process. For this purpose, low temperature cofired ceram-ics substrates were coated over a large area, freely structured and cut withoutmasks by a laser and sintered to a solid structure in a single optimized thermalpost-processing.Ascalabledesignwithcomplexgeometryandlargecoolingsur-face for application on a hot shaft was realized to prove feasibility. Investigationson sintering characteristics up to a peak temperature of 1173K, thermoelec-tric material properties and temperature distribution were carried out for aCa3Co4O9/Ag-based prototype and evaluated using profilometer, XRD, and IRmeasurements. For a combined post-processing, an optimal sintering profilecould be determined at 1073K peak temperature with a 20min holding time.Temperaturegradientsofupto100Kcouldbeachievedalongathermocouple.Asingle TEG module consisting of 12 thermocouples achieved a maximum powerof0.224μWandopen-circuitvoltageof134.41mVatanaveragehot-sidetemper-ature of 413.6 K and temperature difference of 106.7 K. Three of these modulescombined into a common TEG with a total of 36 thermocouples reached a maxi-mumpowerof0.58Kandopen-circuitvoltageof319.28mVwithalesseraveragehot-side temperature of 387.8 K and temperature difference of 83.4 K. |
| Remark |
https://doi.org/10.1002/eng2.12590 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Electrospun Ca3Co4−xO9+δ nanofibers and nanoribbons: Microstructure and thermoelectric properties
| Authors |
|
| Source |
J Am Ceram Soc.
Volume: 106,
Pages: 1170–1181 Time of Publication: 2023 |
| Abstract | Oxide-based ceramics offer promising thermoelectric (TE) materials for recy- cling high-temperature waste heat, generated extensively from industrial sources. To further improve the functional performance of TE materials, their power factor should be increased. This can be achieved by nanostructuring and texturing the oxide-based ceramics creating multiple interphases and nanopores, which simultaneously increase the electrical conductivity and the Seebeck coef- ficient. The aim of this work is to achieve this goal by compacting electrospun nanofibers of calcium cobaltite Ca3 Co 4−xO 9+δ, known to be a promising p-type TE material with good functional properties and thermal stability up to 1200 K in air. For this purpose, polycrystalline Ca3 Co 4−xO 9+δ nanofibers and nanorib- bons were fabricated by sol–gel electrospinning and calcination at intermediate temperatures to obtain small primary particle sizes. Bulk ceramics were formed by sintering pressed compacts of calcined nanofibers during TE measurements. The bulk nanofiber sample pre-calcined at 973 K exhibited an improved Seebeck coefficient of 176.5 S cm−1 and a power factor of 2.47 μW cm−1 K−2 similar to an electrospun nanofiber-derived ceramic compacted by spark plasma sintering. |
| Remark |
DOI: 10.1111/jace.18842 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Tuning the Thermoelectric Performance of CaMnO3-Based Ceramics by Controlled Exsolution and Microstructuring
| Authors |
|
| Source |
CS Appl. Energy Mater.
Volume: 5,
Issue: 10,
Pages: 12396–12407 Time of Publication: 2022 |
| Abstract | The thermoelectric properties of CaMnO3−δ/CaMn2O4 composites were tuned via microstructuring and compositional adjustment. Single-phase rock-salt-structured CaO–MnO materials with Ca:Mn ratios larger than unity were produced in reducing atmosphere and subsequently densified by spark plasma sintering in vacuum. Annealing in air at 1340 °C between 1 and 24 h activated redox-driven exsolution and resulted in a variation in microstructure and CaMnO3−δ materials with 10 and 15 vol % CaMn2O4, respectively. The nature of the CaMnO3−δ/CaMn2O4 grain boundary was analyzed by transmission electron microscopy on short- and long-term annealed samples, and a sharp interface with no secondary phase formation was indicated in both cases. This was further complemented by density functional theory (DFT) calculations, which confirmed that the CaMnO3−δ indeed is a line compound. DFT calculations predict segregation of oxygen vacancies from the bulk of CaMnO3−δ to the interface between CaMnO3−δ and CaMn2O4, resulting in an enhanced electronic conductivity of the CaMnO3−δ phase. Samples with 15 vol % CaMn2O4 annealed for 24 h reached the highest electrical conductivity of 73 S·cm–1 at 900 °C. The lowest thermal conductivity was obtained for composites with 10 vol % CaMn2O4 annealed for 8 h, reaching 0.56 W·m–1K–1 at 700 °C. However, the highest thermoelectric figure-of-merit, zT, was obtained for samples with 15 vol % CaMn2O4 reaching 0.11 at temperatures between 800 and 900 °C, due to the enhanced power factor above 700 °C. This work represents an approach to boost the thermoelectric performance of CaMnO3−δ based composites. |
| Remark |
https://doi.org/10.1021/acsaem.2c02012 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Synthesis of a Novel Nanoparticle BaCoO2.6 through Sol-Gel Method and Elucidation of Its Structure and Electrical Properties
| Authors |
|
| Source |
Journal of Nanomaterials
Time of Publication: 2022
|
| Abstract | The physical properties of cobalt oxide with varied oxidation states, and coordination numbers, in the transition series, have numerous applications. The present study explores the physical properties of BaCoO2.6 nanoparticles synthesized through the sol-gel method. The X-ray diffraction figure exhibits a 25 nm crystallite size hexagonal phase. The observational data shows the reduction in the real part of impedance (), dielectric constant (), dielectric loss (), and a raise in ac conductivity of mixed type of conduction with an elevation in frequency analyzed through impedance spectroscopy. The conductivity due to grain and grain boundaries is shown foremost in the complex impedance analysis. The plot of (Seebeck coefficient) in the low-temperature range indicates p-type behavior and the metal-insulator transition in the as-synthesized sample. The sample characteristics suggest applications in optical and switching devices. The Seebeck coefficient is the generation of potential difference when subjected to temperature difference. Thermoelectric materials are associated with the concept of high electrical conductivity like crystals and low thermal conductivity to that of glass. Nanothermoelectric materials can decrease further the thermal conductivity through phonon scattering. Electrical characterization suggests the presence of both NTCR and PTCR behavior in the sample, and hence, it explores the application in thermistor/resistance temperature detector’s (RTD) and low dielectric constant and loss to electro-optical and higher conversion efficiency to storage devices. Additionally, impedance spectroscopy helps in the study of electrochemical systems and solid-state devices wherein the transition of metal-insulator is an add-on to the research. |
| Remark |
https://doi.org/10.1155/2022/3877879 Ask ChatGPT for Link Ask ChatGPT to Summarize |
La1-xSrxMO3 (M = Co, Mn, Cr) interconnects in a 4-leg all-oxide thermoelectric generator at high temperatures
| Authors |
|
| Source |
Journal of Physics and Chemistry of Solids
Volume: 167,
Pages: 110739 Time of Publication: 2022 |
| Abstract | We herein report tests at high temperatures of a 4-leg oxide thermoelectric generator consisting of two pairs of p-type Ni0.98Li0.02O (Li–NiO) and n-type Zn0.98Al0.02O (Al–ZnO), assembled with various conducting perovskite oxides as interconnects. Using a custom-built testing system, we evaluated performance and stability at a hot side (furnace) temperature of up to 1000 °C under temperature differences up to ΔT = 600 °C in air. With a La0.6Sr0.4CoO3 (LSC) interconnect, a maximum power output of 18 mW was achieved with TH = 940 and TC = 340 °C (ΔT = 600 °C). Power maxima with La0.8Sr0.2MnO3 (LSM) and La0.8Sr0.2CrO3 (LSCr) as interconnects were lower, 6 mW and 2 mW, respectively, under similar conditions, attributed to their lower thermal and electrical conductivities. This demonstrates the requirements and potential of oxide interconnects for stable use of all-oxide thermoelectric generators at high temperatures in ambient air. |
| Keywords | Thermoelectric generator; Oxide; Interconnect; NiO; ZnO; La0.6Sr0.4CoO3 (LSC) |
| Remark |
https://doi.org/10.1016/j.jpcs.2022.110739 Ask ChatGPT for Link Ask ChatGPT to Summarize |
The effect of alkaline earth metal substitution on thermoelectric properties of A0.98La0.02MnO3-δ (A = Ca, Ba)
| Authors |
|
| Source |
Processing and Application of Ceramics
Volume: 16,
Issue: 1,
Pages: 78–82 Time of Publication: 2022 |
| Abstract | The thermoelectric properties of ceramics with composition A0.98La0.02MnO3-δ are anticipated to vary with the basicity and atomic portion of the alkaline earth metal, A. In the present investigation ceramic powder precursors with composition A0.98La0.02MnO3-δ (A = Ca, Ba) were synthesized by the solid-state method and sintered in air at 1400 °C. Seebeck coefficient, electrical and thermal conductivities were characterized for both materials from 100 to 900 °C in air. The highest zT of 0.10 at 900 °C was reached for Ca0.98La0.02MnO3-δ. The high zT is attributed to the enhanced electronic conductivity (∼90 S/cm at 900 °C) due to La doping. zT for Ba0.98La0.02MnO3-δ reached its highest value (0.02) at 800 °C corresponding to a low electronic conductivity (∼2 S/cm), while the thermal conductivity was significantly reduced compared to Ca0.98La0.02MnO3-δ reaching ∼1 W/(m·K) combined with a high Seebeck coefficient, −290 μV/K. The present data represent a valuable basis for further development of these materials with respect to applications in thermoelectric devices. |
| Remark |
https://doi.org/10.2298/PAC2201078S Ask ChatGPT for Link Ask ChatGPT to Summarize |
Lanthanum strontium cobaltite as interconnect in oxide thermoelectric generators
| Authors |
|
| Source |
Solid State Sciences
Volume: 124,
Pages: 106801 Time of Publication: 2022 |
| Abstract | Issues related to use of metallic interconnects in oxide thermoelectric generators (TEGs) need to be addressed to secure performance and durability. Metal interconnects suffer from high cost of noble metals or chemical instability and contact resistance of non-noble metals, arising from oxidation, evaporation, and delamination in the oxidising conditions of ambient air at high operating temperatures. This work introduces the use of a stable and highly conducting ceramic oxide, in our case p-type lanthanum strontium cobaltite (La0.6Sr0.4CoO3, LSC) as interconnect. We verified the thermochemical stability of LSC in contact with p-type Ni0.98Li0.02O (Li–NiO) and n-type Zn0.98Al0.02O (Al–ZnO) and examined the electrical characteristics. An area specific contact resistance (ASRc) of ∼1800 Ω cm2 for a direct p-n junction was reduced to ∼400 mΩ cm2 for a p-LSC-n junction at a temperature of 300 °C, validating the concept. The use of a screen-printed LSC/Al–ZnO composite as a thin interconnect layer was found to decrease the contact resistance of the junction further to ∼260 mΩ cm2 at 300 °C, attributed to increased effective area of the LSC/Al–ZnO p-n junction. |
| Keywords | Thermoelectric generator; All-oxide; Thermoelectric materials; Oxides; Interconnect; Oxide; p-n-junction; Ohmic; LaCoO3; Sr-substituted; La0.6Sr0.4CoO3 |
Reaction Sintering of Ca3Co4O9 with BiCuSeO Nanosheets for High-Temperature Thermoelectric Composites
| Authors |
|
| Source |
Journal of Electronic Materials volume
Volume: 51,
Pages: 532–542 Time of Publication: 2022 |
| Abstract | Ceramic composites composed of oxide materials have been synthesized by reaction sintering of Ca3Co4O9 with BiCuSeO nanosheets. In situ x-ray diffraction and thermogravimetric analyses of the compound powders were conducted to understand the phase transformations during heating up to 1173 K. Further thermogravimetric analyses investigated the thermal stability of the composites and the completion of reaction sintering. The microstructure of the formed phases after reaction sintering and the composition of the composites were investigated for varying mixtures. Depending on the amount of BiCuSeO used, the phases present and their composition differed, having a significant impact on the thermoelectric properties. The increase of the electrical conductivity at a simultaneously high Seebeck coefficient resulted in a large power factor of 5.4 μW cm−1 K−2, more than twice that of pristine Ca3Co4O9. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Improved thermoelectric properties in ceramic composites based on Ca3Co4O9 and Na2Ca2Nb4O13
| Authors |
|
| Source |
Open Ceramics
Volume: 8,
Pages: 100198 Time of Publication: 2021 |
| Abstract | The oxide materials Ca3Co4O9 and Na2Ca2Nb4O13 were combined in a new ceramic composite with promising synergistic thermoelectric properties. Both compounds show a plate-like crystal shape and similar aspect ratios but the matrix material Ca3Co4O9 with lateral sizes of less than 500 nm is about two orders of magnitude smaller. Uniaxial pressing of the mixed compound powders was used to produce porous ceramics after conventional sintering. Reactions between both compounds and their compositions were thoroughly investigated. In comparison to pure Ca3Co4O9, mixing with low amounts of Na2Ca2Nb4O13 proved to be beneficial for the overall thermoelectric properties. A maximum figure-of-merit of zT = 0.32 at 1073 K and therefore an improvement of about 19% was achieved by the ceramic composites. |
| Remark |
https://doi.org/10.1016/j.oceram.2021.100198 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Glass-ceramic composites as insulation material for thermoelectric oxide multilayer generators
| Authors |
|
| Source |
Time of Publication: 2021
|
| Abstract | Thermoelectric generators can be used as energy harvesters for sensor applications. Adapting the ceramic multilayer technology, their production can be highly automated. In such multilayer thermoelectric generators, the electrical insulation material, which separates the thermoelectric legs, is crucial for the performance of the device. The insulation material should be adapted to the thermoelectric regarding its averaged coefficient of thermal expansion α and its sintering temperature while maintaining a high resistivity. In this study, starting from theoretical calculations, a glass-ceramic composite material adapted for multilayer generators from calcium manganate and calcium cobaltite is developed. The material is optimized towards an α of 11 × 10−6 K−1 (20–500°C), a sintering temperature of 900°C, and a high resistivity up to 800°C. Calculated and measured α are in good agreement. The chosen glass-ceramic composite with 45 vol.% quartz has a resistivity of 1 × 107 Ωcm and an open porosity of <3%. Sintered multilayer samples from tape-cast thermoelectric oxides and screen-printed insulation show only small reaction layers. It can be concluded that glass-ceramic composites are a well-suited material class for insulation layers as their physical properties can be tuned by varying glass composition or dispersion phases. |
| Remark |
https://doi.org/10.1111/jace.18235 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Effect of ball-milling on the phase formation and enhanced thermoelectric properties in zinc antimonides
| Authors |
|
| Source |
Materials Science and Engineering: B
Volume: 271,
Pages: 115274 Time of Publication: 2021 |
| Abstract | We report the phase formation mechanism and the enhanced thermoelectric properties of zinc antimonide (ZnSb) thermoelectric material. The phase pure ZnSb thermoelectric material is achieved using high-energy ball milling of Zn and Sb in a shorter span of time. The ZnSb phase formation is explained by the kinetic energy transferred to the powders during milling for the solid-state reaction between Zn and Sb to form the desired ZnSb phase. The repeatability in transport properties up to three thermal cycles corroborates the thermal stability of the processed samples. The thermoelectric figure of merit obtained at 600 K is ~ 0.76 for the processed phase pure ZnSb sample, the highest value in binary ZnSb reported so far. Our results address the ZnSb phase evolution in a shorter milling time and the enhanced thermoelectric properties of the ZnSb materials. The observations will help to scale up the processing of high-performance ZnSb thermoelectric materials. |
| Keywords | Zinc antimonide; Thermoelectric materials; Ball milling; Phase formation kinetics; X-ray diffraction; Figure of merit |
| Remark |
https://doi.org/10.1016/j.mseb.2021.115274 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Influence of Doping on the Transport Properties of Y1−xLnxMnO3+δ (Ln: Pr, Nd)
| Author |
|
| Source |
Crystals
Volume: 11,
Pages: 510 Time of Publication: 2021 |
| Abstract | It has been documented that the total electrical conductivity of the hexagonal rare-earth manganites Y0.95Pr0.05MnO3+δ and Y0.95Nd0.05MnO3+δ, as well as the undoped YMnO3+δ, is largely dependent on the oxygen excess δ, which increases considerably at temperatures below ca. 300 âŠC in air or O2. Improvement for samples maintaining the same P63cm crystal structure can exceed 3 orders of magnitude below 200 âŠC and is related to the amount of the intercalated oxygen. At the same time, doping with Nd3+ or Pr3+ affects the ability of the materials to incorporate O2, and therefore indirectly influences the conductivity as well. At high temperatures (700–1000 âŠC) and in different atmospheres of Ar, air, and O2, all materials are nearly oxygen-stoichiometric, showing very similar total conduction with the activation energy values of 0.8–0.9 eV. At low temperatures in Ar (δ ≈ 0), the mean ionic radius of Y1−xLnx appears to influence the electrical conductivity, with the highest values observed for the parent YMnO3. For Y0.95Pr0.05MnO3+δ oxide, showing the largest oxygen content changes, the recorded dependence of the Seebeck coefficient on the temperature in different atmospheres exhibits complex behavior, reflecting oxygen content variations, and change of the dominant charge carriers at elevated temperatures in Ar (from electronic holes to electrons). Supplementary cathodic polarization resistance studies of the Y0.95Pr0.05MnO3+δ electrode document different behavior at higher and lower temperatures in air, corresponding to the total conduction characteristics. |
Fabrication of a Silicide Thermoelectric Module Employing Fractional Factorial Design Principles
| Authors |
|
| Source |
Journal of Electronic Materials volume
Volume: 50,
Pages: 4041–4049 Time of Publication: 2021 |
| Abstract | Thermoelectric modules can be used in waste heat harvesting, sensing, and cooling applications. Here, we report on the fabrication and performance of a four-leg module based on abundant silicide materials. While previously optimized Mg2Si0.3Sn0.675Bi0.025 is used as the n-type leg, we employ a fractional factorial design based on the Taguchi methods mapping out a four-dimensional parameter space among Mnx-εMoεSi1.75−δGeδ higher manganese silicide compositions for the p-type material. The module is assembled using a scalable fabrication process, using a Cu metallization layer and a Pb-based soldering paste. The maximum power output density of 53 μW cm–2 is achieved at a hot-side temperature of 250 °C and a temperature difference of 100 °C. This low thermoelectric output is related to the high contact resistance between the thermoelectric materials and the metallic contacts, underlining the importance of improved metallization schemes for thermoelectric module assembly. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Ceramic composites based on Ca3Co4−xO9+δ and La2NiO4+δ with enhanced thermoelectric properties
| Authors |
|
| Source |
Open Ceramics
Volume: 6,
Pages: 100103 Time of Publication: 2021 |
| Abstract | Ceramic composites were produced by combining the oxide materials Ca3Co4−xO9+δ and La2NiO4+δ. Both compounds were characterized by a plate-like crystal shape, but crystal sizes differed by around two orders of magnitude. The composite materials could be successfully prepared by using uniaxial pressing of powder mixtures and pressureless sintering to a porous ceramic. Possible reactions between both materials during sintering were analyzed. The ceramic composites with low amounts of La2NiO4+δ showed enhanced thermoelectric properties, caused by an increasing power factor and simultaneously decreasing thermal conductivity. For the evaluation of the thermoelectric properties, two different types of Ioffe plots were utilized. The maximum figure-of-merit zT at 1073 âK was 0.27 for the pure Ca3Co4−xO9+δ as well as for the sample containing 5 âwt% La2NiO4+δ. However, the average in the temperature range of 373 K to 1073 K could be increased by 20% for the composite material. |
| Keywords | Calcium cobalt oxide; Composite; Ceramic; Lanthanum nickelate; Reaction sintering; Thermoelectric; Power factor; Figure-of-merit |
Versatile four-leg thermoelectric module test setup adapted to a commercial sample holder system for high temperatures and controlled atmospheres
| Authors |
|
| Source |
Review of Scientific Instruments
Volume: 92,
Pages: 043902 Time of Publication: 2021 |
| Abstract | A high temperature thermoelectric test setup for the NORECS ProboStat™ sample holder cell has been designed, constructed, and tested. It holds four thermoelectric legs of up to 5 × 5 mm2 area each and flexible height, allows various interconnects to be tested, and utilizes the spring-load system of the ProboStat for fixation and contact. A custom stainless steel support tube flushed with water provides the cold sink, enabling large temperature gradients. Thermocouples and electrodes as well as the gas supply and outer tube use standard ProboStat base unit feedthroughs and dimensions. The setup allows for testing in controlled atmospheres with the hot side temperature of up to around 1000 °C and a temperature gradient of up to 600 °C. We demonstrate the test setup on a four-leg Li–NiO/Al–ZnO module with gold interconnects. The comparison between the predicted performance based on individual material parameters and the experimentally obtained module performance underlines the necessity for testing materials in combination, including interconnects. The four-leg setup allows versatile match-screening, performance evaluation, and long-term stability studies of thermoelectric materials in combination with hot and cold side interconnects under realistic operational conditions. |
Thermal Conductivity and Thermoelectric Power of Compounds in the Cu–Ge–As–Se System
| Authors |
|
| Source |
Technical Physics volume
Volume: 66,
Pages: 41–45 Time of Publication: 2021 |
| Abstract | The influence of temperature (in the interval of 300–400 K) and concentrations on the electrical conductivity, thermal conductivity, and thermoelectric power of copper chalcogenide-based crystals with the general formula (GeSe)1 – x(CuAsSe2)x has been considered. Heat transfer mechanisms have been determined. It has been found that the temperature dependence of thermal conductivity is nonmonotonic with a singularity at 358 K. Thermoelectric figure of merit ZT has been calculated. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Near-Broken-Gap Alignment between FeWO4 and Fe2WO6 for Ohmic Direct p–n Junction Thermoelectrics
| Authors |
|
| Source |
ACS Appl. Mater. Interfaces
Volume: 13,
Issue: 6,
Pages: 7416–7422 Time of Publication: 2021 |
| Abstract | We report a near-broken-gap alignment between p-type FeWO4 and n-type Fe2WO6, a model pair for the realization of Ohmic direct junction thermoelectrics. Both undoped materials have a large Seebeck coefficient and high electrical conductivity at elevated temperatures, due to inherent electronic defects. A band-alignment diagram is proposed based on X-ray photoelectron and ultraviolet–visible light reflectance spectroscopy. Experimentally acquired nonrectifying I–V characteristics and the constructed band-alignment diagram support the proposed formation of a near-broken-gap junction. We have additionally performed computational modeling based on density functional theory (DFT) on bulk models of the individual compounds to rationalize the experimental band-alignment diagram and to provide deeper insight into the relevant band characteristics. The DFT calculations confirm an Fe-3d character of the involved band edges, which we suggest is a decisive feature for the unusual band overlap. |
| Keywords | thermoelectric oxides, broken-gap junction, Ohmic contact, band alignment, p-n junction, computational first-principles modeling |
| Remark |
https://doi.org/10.1021/acsami.0c19341 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Improved environmental stability of thermoelectric ceramics based on intergrowths of Ca3Co4O9–Na0.75CoO2
| Authors |
|
| Source |
Ceramics International
Volume: 47,
Issue: 8,
Pages: 11687-11693 Time of Publication: 2021 |
| Abstract | Ceramics based on calcium and sodium cobaltates are promising high-temperature thermoelectric oxide materials with complementary advantages. Ca3Co4O9 is stable at high temperatures, whereas Na0.75CoO2 can be easily processed as a textured ceramic with excellent thermoelectric properties. Na0.75CoO2, however, lacks long-term stability and degrades in even a relatively mild humid environment. In this work, we present a novel approach to the synthesis of complex composite materials based on intergrowths of sodium and calcium cobaltates that have excellent thermoelectric performance and improved stability. We synthesized samples with the mixed composition (3-x)Ca3Co4O9–4x(Na0.75CoO2) in an over-pressured oxygen atmosphere. Samples with the mixed Ca–Na composition developed textured microstructures composed of intergrowths of both end-members, as revealed by transmission electron microscopy. We also examined the thermoelectric performance of the investigated materials after exposure to high humidity and found that the composition with x = 0.8 (Ca:Na = 2.75) has long-term stability. |
| Keywords | Composite materials; Microstructure; Transmission electron microscopy; Thermoelectric |
Time-Enhanced Performance of Oxide Thermoelectric Modules Based on a Hybrid p–n Junction
| Authors |
|
| Source |
ACS Omega
Volume: 6,
Issue: 1,
Pages: 197–205 Time of Publication: 2020 |
| Abstract | The present challenge with all-oxide thermoelectric modules is their poor durability at high temperatures caused by the instability of the metal-oxide interfaces at the hot side. This work explains a new module concept based on a hybrid p–n junction, fabricated in one step by spark plasma co-sintering of Ca3Co4–xO9+δ (CCO, p-type) and CaMnO3−δ/CaMn2O4 (CMO, n-type). Different module (unicouple) designs were studied to obtain a thorough understanding of the role of the in situ formed hybrid p–n junction of Ca3CoMnO6 (CCMO, p-type) and Co-oxide rich phases (p-type) at the p–n junction (>700 °C) in the module performance. A time-enhanced performance of the modules attributed to this p–n junction formation was observed due to the unique electrical properties of the hybrid p–n junction being sufficiently conductive at high temperatures (>700 °C) and nonconductive at moderate and low temperatures. The alteration of module design resulted in a variation of the power density from 12.4 (3.1) to 28.9 mW/cm2 (7.2 mW) at ΔT ∼ 650 °C after 2 days of isothermal hold (900 °C hot side). This new concept provides a facile method for the fabrication of easily processable, cheap, and high-performance high-temperature modules. |
Thermally stable, low resistance Mg2Si0.4Sn0.6/Cu thermoelectric contacts using SS 304 interlayer by one step sintering
| Authors |
|
| Source |
Materials Research Bulletin
Volume: 136,
Pages: 111147 Time of Publication: 2021 |
| Abstract | Device fabrication using Mg2Si1-xSnx thermoelectric (TE) material for 600–800 K application requires stable and low resistance electrical contacts between TE legs and the electrodes. In this study, n-type Mg2Si0.38Sn0.6Bi0.02 was hot-pressed with Cu electrodes in a single step, resulting in Cu3Mg2Si and Cu4MgSn phases at the interface. Although the specific contact resistance (rc) across the interface was 4.4 ± 0.9 μΩ.cm2, the electrical resistivity of the TE leg increased by approximately 60 % due to Cu diffusion through the interface. Incorporating the SS304 interlayer to prevent Cu diffusion increased rc to 6.1 ± 2 μΩ.cm2. Upon annealing at 723 K for 3–15 days, rc remained at <10 μΩ.cm2 with an approximately 15 % decrease in the power factor. However, without SS304, rc increased to 41.5 ± 18 μΩ.cm2, with 65 % reduction in the power factor. Thus, this work demonstrates the fabrication of thermally stable Cu/Mg2Si0.4Sn0.6 joints by using the SS304 interlayer in a single-step process. |
Defects and polaronic electron transport in Fe2WO6
| Authors |
|
| Source |
Physical Chemistry Chemical Physics
Issue: 27
Time of Publication: 2020
|
| Abstract | We report the synthesis of phase pure Fe2WO6 and its structural characterization by high quality synchrotron X-ray powder diffraction, followed by studies of electric and thermoelectric properties as a function of temperature (200–950 °C) and pO2 (1–10−3 bar). The results are shown to be in accordance with a defect chemical model comprising formation of oxygen vacancies and charge compensating electrons at high temperatures. The standard enthalpy and entropy of formation of an oxygen vacancy and two electrons in Fe2WO6 are found to be 113(5) kJ mol−1 and 41(5) J mol−1 K−1, respectively. Electrons residing as Fe2+ in the Fe3+ host structure act as charge carriers in a small polaron conducting manner. A freezing-in of oxygen vacancies below approximately 650 °C results in a region of constant charge carrier concentration, corresponding to an iron site fraction of XFe2+ ≅ 0.03. By decoupling of mobility from conductivity, we find a polaron hopping activation energy of 0.34(1) eV and a charge mobility pre-exponential u0 = 400(50) cm2 kV−1 s−1. We report thermal conductivity for the first time for Fe2WO6. The relatively high conductivity, large negative Seebeck coefficient and low thermal conductivity make Fe2WO6 an interesting candidate as an n-type thermoelectric in air, for which we report a maximum zT of 0.027 at 900 °C. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Ceramic-based thermoelectric generator processed via spray-coating and laser structuring
| Authors |
|
| Source |
Open Ceramics
Volume: 1,
Pages: 100002 Time of Publication: 2020 |
| Abstract | Processing technology to improve the manufacturing of thermoelectric generators (TEGs) is a growing field of research. In this paper, an adaptable and scalable process comprising spray-coating and laser structuring for fast and easy TEG manufacturing is presented. The developed process combines additive and subtractive processing technology towards an adaptable ceramic-based TEG, which is applicable at high temperatures and shows a high optimization potential. As a prototype, a TEG based on Ca3Co4O9 (CCO) and Ag on a ceramic substrate was prepared. Microstructural and thermoelectric characterization is shown, reaching up to 1.65 âμW âcm−2 at 673 âK and a ΔT of 100 âK. The high controllability of the developed process also enables adaptation for different kinds of thermoelectric materials. |
Tuning of Mg content to enhance the thermoelectric properties in binary Mg2+δ Si (δ = 0, 0.1, 0.15, 0.2)
| Authors |
|
| Source |
Materials Research Express
Volume: 6
Time of Publication: 2019
|
| Abstract | We report the enhanced thermoelectric properties of binary Mg2Si by tuning the Mg content. Polycrystalline Mg2+δ Si (where δ is the excess Mg content in the starting composition of the samples and δ = 0, 0.1, 0.15, 0.2) samples were processed by solid-state synthesis route using ball milling followed by rapid spark plasma sintering in order to minimize the Mg loss during processing. Microstructural and x-ray diffraction analysis revealed that, Mg content (δ) of 0.1–0.15 is required to get the binary Mg2Si phase without any elemental Mg/Si phase. Hall effect measurement and Fourier Transform Infrared Spectroscopy analysis show that, the excess Mg content helps to enhance the carrier concentration and charge carrier effective mass due to the occupancy of Mg at the interstitial site in Mg2Si structure. The influence of Mg content on thermoelectric properties, viz., electrical resistivity, Seebeck coefficient and thermal conductivity is investigated from 300 K to 780 K. A marked enhancement in thermoelectric power factor (~1.6 mW m−2K−2) is obtained for Mg2.15Si sample at 780 K. The occupancy of excess Mg at interstitial sites reduces the lattice thermal conductivity by lowering lattice symmetry. A maximum figure of merit (ZT) ~ 0.39 ± 0.03 at 780 K has been achieved in Mg2.15Si sample, the highest among that reported in n-type binary Mg2Si system. This suggests that excess Mg content in the starting composition of Mg2+δ Si helps in stabilizing the phase as well as improves the thermoelectric properties of the Mg2Si. |
| Remark |
https://doi.org/10.1088/2053-1591/ab58fb Ask ChatGPT for Link Ask ChatGPT to Summarize |
Thermoelectric properties of A-site deficient La-doped SrTiO3 at 100–900 °C under reducing conditions
| Authors |
|
| Source |
Journal of the European Ceramic Society
Volume: 40,
Issue: 2,
Pages: 401-407 Time of Publication: 2020 |
| Abstract | Lanthanum doped strontium titanate is a potential n-type thermoelectric material at moderate and high temperatures. (La0.12Sr0.88)0.95TiO3 ceramics were prepared by two different routes, conventional sintering at 1500 °C and spark plasma sintering at temperatures between 925 and 1200 °C. Samples with grain size between 40 nm and 1.4 μm were prepared and characterized with respect to their thermoelectric transport properties at temperatures between 100 and 900 °C under reducing conditions (H2/H2O-buffer mixtures). The thermal conductivity was significantly reduced with decreasing grain size reaching a value of 1.3 W m−1. K−1 at 600 °C for grain size of 40 nm and porosity of 19%. Electrical conductivity increased with increasing grain size showing a maximum of 500 S cm−1 at 200 °C for a grain size of 1.4 μm. The highest figure-of-merit (zT) was measured for samples with 1.4 μm average grain size reaching 0.2 at 500 °C. |
| Remark |
https://doi.org/10.1016/j.jeurceramsoc.2019.09.024 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Metal oxides for thermoelectrics
| Author |
|
| Source |
Time of Publication: 2019
|
| Remark |
Dissertation Ask ChatGPT for Link Ask ChatGPT to Summarize |
AlTiN based thin films for degradation protection of tetrahedrite thermoelectric material
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 792,
Pages: 953-959 Time of Publication: 2019 |
| Abstract | Efficient protection against degradation process of tetrahedrite-based thermoelectric materials was obtained employing AlTiN based thin films. The coatings were deposited via reactive direct current physical vapour deposition magnetron sputtering. The composition, thermal and electrical behaviour of thin films were investigated by X-ray diffraction, energy dispersive spectroscopy associated to field emission scanning electron microscopy, thermogravimetric analyses and electrical conductivity measurements. The barrier features for oxygen protection during thermal treatment in air at 500 °C were qualitatively evaluated, studying the coating behaviour over the higher operating temperature of tetrahedrite based thermoelectric devices. |
| Remark |
https://doi.org/10.1016/j.jallcom.2019.04.116 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Tuning the optical and thermoelectric properties of SrTi0.8−x Sn0.2FexO3
| Authors |
|
| Source |
Materials Research Express
Volume: 6,
Issue: 4
Time of Publication: 2019
|
| Abstract | Effect of Fe doping on the structure, optical and thermoelectric properties of SrTi0.8Sn0.2O3 sample has been investigated. The SrTi0.8−xSn0.2FexO3 (x = 0, 0.1, 0.3) samples are fabricated using solid-state synthesis route. It is observed that Fe doping helps in reducing the densification temperature of SrTi0.8Sn0.2O3 during spark plasma sintering. Precipitation of Sn has been observed in SrTi0.8−xSn0.2FexO3 (x = 0, 0.1) samples while the SrTi0.8−xSn0.2FexO3 (x = 0.3) sample is of purely single cubic perovskite phase. All the samples consist of nanocrystalline grains and the grain size varies between 150 to 200 nm. Fourier transform infrared spectroscopy (FTIR) analysis reveals the distortion of TiO6 octahedra due to the increase in Fe content. Raman spectroscopy analysis has shown that perovskite cubic structure is stable from room temperature to 873 K. From thermophysical measurements, it is shown that the Fermi band gap reduces from 2.87 to 0.66 eV with increase in Fe in the investigated samples. The Seebeck co-efficient is found to change the sign from n –type to p-type with the increase of Fe concentration in SrTi0.8Sn0.2O3, which is an interesting observation to obtain p-type SrTiO3 based thermoelectric materials. The optical and thermoelectric properties show that Fe doping improves the thermoelectric properties of SrTi0.8Sn0.2O3 ceramics by altering the Seebeck co-efficient and thermal conductivity. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Large-area and adaptable electrospun silicon-based thermoelectric nanomaterials with high energy conversion efficiencies
| Authors |
|
| Source |
Nature Communications
Volume: 9
Time of Publication: 2018
|
| Abstract | Large amounts of waste heat generated in our fossil-fuel based economy can be converted into useful electric power by using thermoelectric generators. However, the low-efficiency, scarcity, high-cost and poor production scalability of conventional thermoelectric materials are hindering their mass deployment. Nanoengineering has proven to be an excellent approach for enhancing thermoelectric properties of abundant and cheap materials such as silicon. Nevertheless, the implementation of these nanostructures is still a major challenge especially for covering the large areas required for massive waste heat recovery. Here we present a family of nano-enabled materials in the form of large-area paper-like fabrics made of nanotubes as a cost-effective and scalable solution for thermoelectric generation. A case study of a fabric of p-type silicon nanotubes was developed showing a five-fold improvement of the thermoelectric figure of merit. Outstanding power densities above 100 W/m2 at 700 °C are therefore demonstrated opening a market for waste heat recovery. |
| Remark |
Article number: 4759 (2018) Ask ChatGPT for Link Ask ChatGPT to Summarize |
A comprehensive study on improved power materials for high-temperature thermoelectric generators
| Authors |
|
| Source |
Journal of Power Sources
Volume: 410-411,
Pages: 143-151 Time of Publication: 2019 |
| Abstract | Dense Ca3Co4O9-NaxCoO2-Bi2Ca2Co2O9 (CCO-NCO-BCCO) nanocomposites were produced from sol-gel derived Ca2.25Na0.3Bi0.35Tb0.1Co4O9 powder by four methods: Hot-pressing (HP), spark plasma sintering (SPS) and pressureless sintering in air or O2 atmosphere. Nanocomposites from HP and SPS revealed nanosized grains and showed a thermoelectric power factor of 4.8 and 6.6 μW cm−1 K−2, respectively, at 1073 K in air. A dense 2D nanocomposite with structures on multiple length scales and enhanced thermoelectric properties was obtained from pressureless sintering in O2 atmosphere. The resulting 2D nanocomposite enabled the simultaneous increase in isothermal electrical conductivity σ and Seebeck coefficient α, and showed a thermoelectric power factor of 8.2 μW cm−1 K−2 at 1073 K in air. The impact of materials with enhanced electrical conductivity and power factor on the electrical power output of thermoelectric generators was verified in prototypes. A high electrical power output and power density of 22.7 mW and 113.5 mW cm−2, respectively, were obtained, when a hot-side temperature of 1073 K and a temperature difference of 251 K were applied. Different p- and n-type materials were used to verify the effect of the thermoelectric figure-of-merit and power factor on the performance of thermoelectric generators. |
Triple-phase ceramic 2D nanocomposite with enhanced thermoelectric properties
| Authors |
|
| Source |
Journal of the European Ceramic Society
Volume: 39,
Issue: 4,
Pages: 1237-1244 Time of Publication: 2019 |
| Abstract | A thermoelectric triple-phase p-type Ca3Co4O9-NaxCoO2-Bi2Ca2Co2O9 (CCO–NCO–BCCO) 2D nanocomposite was obtained from pressureless sintering in air. The anisotropic thermoelectric properties of the nanocomposite exhibit a high electrical conductivity of 116 S cm−1 and a power factor of 6.5 μW cm−1 K−2 perpendicular to the pressing direction at 1073 K in air. A corresponding zT value of 0.35 was obtained. Three co-doped, thermoelectrically active misfit-layered materials were stacked to form a triple-phase nanocomposite, which combines the advantages of all three materials. The resulting nanocomposite enables simultaneous increases of the isothermal electrical conductivity σ and the Seebeck coefficient α by charge carrier concentration engineering and synergistic effects. The Bi2Ca2Co2O9 and NaxCoO2 phases were stabilized in a Ca3Co4O9 matrix at high temperatures. To evaluate the application of the nanocomposite in high-temperature thermoelectric generators, the representation of the electrical conductivity and power factor in a Ioffe plot was more appropriate than the zT value. |
Computational Prediction and Experimental Realization of p-Type Carriers in the Wide-Band-Gap Oxide SrZn1–xLixO2
| Authors |
|
| Source |
Inorg. Chem.
Volume: 57,
Issue: 19,
Pages: 11874-11883 Time of Publication: 2018 |
| Abstract | It is challenging to achieve p-type doping of zinc oxides (ZnO), which are of interest as transparent conductors in optoelectronics. A ZnO-related ternary compound, SrZnO2, was investigated as a potential host for p-type conductivity. First-principles investigations were used to select from a range of candidate dopants the substitution of Li+ for Zn2+ as a stable, potentially p-type, doping mechanism in SrZnO2. Subsequently, single-phase bulk samples of a new p-type-doped oxide, SrZn1–xLixO2 (0 < x < 0.06), were prepared. The structural, compositional, and physical properties of both the parent SrZnO2 and SrZn1–xLixO2 were experimentally verified. The band gap of SrZnO2 was calculated using HSE06 at 3.80 eV and experimentally measured at 4.27 eV, which confirmed the optical transparency of the material. Powder X-ray diffraction and inductively coupled plasma analysis were combined to show that single-phase ceramic samples can be accessed in the compositional range x < 0.06. A positive Seebeck coefficient of 353(4) μV K–1 for SrZn1–xLixO2, where x = 0.021, confirmed that the compound is a p-type conductor, which is consistent with the pO2 dependence of the electrical conductivity observed in all SrZn1–xLixO2 samples. The conductivity of SrZn1–xLixO2 is up to 15 times greater than that of undoped SrZnO2 (for x = 0.028 σ = 2.53 μS cm–1 at 600 °C and 1 atm of O2). |
Microstructure and doping effect on the enhancement of the thermoelectric properties of Ni doped Dy filled CoSb3 skutterudites
| Authors |
|
| Source |
Sustainable Energy Fuels
Volume: 2,
Pages: 2687-2697 Time of Publication: 2018 |
| Abstract | The thermoelectric properties of nanostructured Ni doped Dy filled CoSb3 skutterudites (Dy0.4Co4−xNixSb12 (x = 0, 0.4, and 0.8)) have been reported. The samples are processed using a solid-state synthesis route. The structural analysis of the samples using X-ray diffraction reveals the existence of a single skutterudite phase in Ni doped samples irrespective of the Ni concentration. Microstructure studies using transmission electron microscopy and scanning electron microscopy show the existence of nanometer (∼60 nm) size equiaxed grains in the investigated samples. A few recrystallized elongated grains (∼200 nm) are observed in the Dy0.4Co3.2Ni0.8Sb12 sample. The power factor of the Dy0.4Co3.2Ni0.8Sb12 sample is enhanced to 5.2 mW mK−2, which is the highest power factor for the doped ternary skutterudites reported so far. The enhancement of the power factor is due to the substantial reduction in electrical resistivity with an increase in Ni concentration at higher temperature. The lattice thermal conductivity is drastically reduced to 0.3 W mK−1 at 773 K in the Dy0.4Co3.2Ni0.8Sb12 sample due to the enhanced phonon scattering from Ni induced point defects and grain boundaries. As a result, a huge increase in the figure of merit (ZT ∼ 1.4 ± 0.14) at 773 K is observed in the Dy0.4Co3.2Ni0.8Sb12 sample, the highest among those of the single element filled CoSb3 skutterudites reported so far at this temperature. Hence, Ni doping could enhance the thermoelectric efficiency of Dy filled CoSb3 skutterudites. This can be taken as a reference to synthesize CoSb3 skutterudite thermoelectric materials having a higher figure of merit. |
| Remark |
DOI: 10.1039/C8SE00395E Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Computational Prediction and Experimental Realization of p-Type Carriers in the Wide-Band-Gap Oxide SrZn1–xLixO2
| Authors |
|
| Source |
Inorg. Chem.
Time of Publication: 2018
|
| Abstract | It is challenging to achieve p-type doping of zinc oxides (ZnO), which are of interest as transparent conductors in optoelectronics. A ZnO-related ternary compound, SrZnO2, was investigated as a potential host for p-type conductivity. First-principles investigations were used to select from a range of candidate dopants the substitution of Li+ for Zn2+ as a stable, potentially p-type, doping mechanism in SrZnO2. Subsequently, single-phase bulk samples of a new p-type-doped oxide, SrZn1–xLixO2 (0 < x < 0.06), were prepared. The structural, compositional, and physical properties of both the parent SrZnO2 and SrZn1–xLixO2 were experimentally verified. The band gap of SrZnO2 was calculated using HSE06 at 3.80 eV and experimentally measured at 4.27 eV, which confirmed the optical transparency of the material. Powder X-ray diffraction and inductively coupled plasma analysis were combined to show that single-phase ceramic samples can be accessed in the compositional range x < 0.06. A positive Seebeck coefficient of 353(4) μV K–1 for SrZn1–xLixO2, where x = 0.021, confirmed that the compound is a p-type conductor, which is consistent with the pO2 dependence of the electrical conductivity observed in all SrZn1–xLixO2 samples. The conductivity of SrZn1–xLixO2 is up to 15 times greater than that of undoped SrZnO2 (for x = 0.028 σ = 2.53 μS cm–1 at 600 °C and 1 atm of O2). |
| Remark |
DOI: 10.1021/acs.inorgchem.8b00697 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Thermoelectric Properties of (1-x)LaCoO3.(x)La0.95Sr0.05CoO3 composite
| Authors |
|
| Source |
Materials Research Express
Time of Publication: 2018
|
| Abstract | Thermopower in cobalt oxides has been a rich area of interest due to the existence of the different charge states along-with different spin states. In this report, we have systematically studied the structural and thermal transport properties of ($1-x$)LaCoO$_3$.($x$)La$_{0.95}$Sr$_{0.05}$CoO$_3$ composite. The Seebeck coefficient ($alpha$) values for the composite increases at high temperatures compared to the LaCoO$_3$ (LCO) and La$_{0.95}$Sr$_{0.05}$CoO$_3$ (LSCO) systems. The electrical conductivity ($sigma$) decreases with the increase in the LSCO fraction which may be attributed to the localization of charge carriers due to intersite diffusion. All the samples show increase in the value of $sigma$ with increase in temperature. The thermal conductivity ($kappa$) values decrease with the increase of LSCO content in the composite and the phonon thermal conductivity dominates over the total thermal conductivity. We observe a maximum value of figure of merit (ZT)$sim$0.06 at 640,K for $x=$0.05. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
All-Oxide Thermoelectric Module with in Situ Formed Non-Rectifying Complex p–p–n Junction and Transverse Thermoelectric Effect
| Authors |
|
| Source |
ACS Omega
Volume: 3,
Issue: 8,
Pages: 9899–9906 Time of Publication: 2018 |
| Abstract | All-oxide thermoelectric modules for energy harvesting are attractive because of high-temperature stability, low cost, and the potential to use nonscarce and nontoxic elements. Thermoelectric modules are mostly fabricated in the conventional π-design, associated with the challenge of unstable metallic interconnects at high temperature. Here, we report on a novel approach for fabrication of a thermoelectric module with an in situ formed p–p–n junction made of state-of-the-art oxides Ca3Co4–xO9+δ (p-type) and CaMnO3–CaMn2O4 composite (n-type). The module was fabricated by spark plasma co-sintering of p- and n-type powders partly separated by insulating LaAlO3. Where the n- and p-type materials originally were in contact, a layer of p-type Ca3CoMnO6 was formed in situ. The hence formed p–p–n junction exhibited Ohmic behavior and a transverse thermoelectric effect, boosting the open-circuit voltage of the module. The performance of the module was characterized at 700–900 °C, with the highest power output of 5.7 mW (around 23 mW/cm2) at 900 °C and a temperature difference of 160 K. The thermoelectric properties of the p- and n-type materials were measured in the temperature range 100–900 °C, where the highest zT of 0.39 and 0.05 were obtained at 700 and 800 °C, respectively, for Ca3Co4–xO9+δ and the CaMnO3–CaMn2O4 composite. |
| Remark |
DOI: 10.1021/acsomega.8b01357 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Thermoelectric properties of (1-x)LaCoO3.xLa0.7Sr0.3MnO3 composite
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 749,
Pages: 1092-1097 Time of Publication: 2018 |
| Abstract | We report the thermoelectric (TE) properties of (1-x)LaCoO3.xLa0.7Sr0.3MnO3 (0 < x < 0.50) composite in a temperature range 320–800 K. Addition of La0.7Sr0.3MnO3 to LaCoO3 in small amount (5 weight %) improves the overall Seebeck coefficient (α) at higher temperatures. The electrical conductivity however decreases due to a decrease in carrier concentration of the composite. The decrease in electrical conductivity of the composite at high temperature may be attributed to the insulating nature of the LSMO above room temperature. Thermal conductivity (κ) of all the samples increases with an increase in the temperature, but decreases with increasing LSMO content. We also report the local variation of Seebeck coefficient across the composite samples measured using a precision Seebeck measurement system. A maximum value of 0.09 for the figure of merit (ZT) is obtained for 0.95LaCoO3.0.05La0.7Sr0.3MnO3 at 620 K which is significantly higher than the ZT of either of LaCoO3 or La0.7Sr0.3MnO3 at 620 K. This suggests the potential for enhancement of operating temperatures of hitherto well known low temperature thermoelectric materials through suitable compositing approach. |
| Keywords | Thermal conductivity, Electrical conductivity, Perovskites, Manganites, Cobaltate, Composite |
| Remark |
https://doi.org/10.1016/j.jallcom.2018.03.347 Ask ChatGPT for Link Ask ChatGPT to Summarize |
p-Type/n-type behaviour and functional properties of KxNa(1-x)NbO3 (0.49 ≤ x ≤ 0.51) sintered in air and N2
| Authors |
|
| Source |
Journal of the European Ceramic Society
Volume: 38,
Issue: 9,
Pages: 3118-3126 Time of Publication: 2018 |
| Abstract | Potassium sodium niobate (KNN) is a potential candidate to replace lead zirconate titanate in sensor and actuator applications but there are many fundamental science and materials processing issues to be understood before it can be used commercially, including the influence of composition and processing atmosphere on the conduction mechanisms and functional properties. Consequently, KNN pellets with different K/Na ratios were sintered to 95% relative density in air and N2 using a conventional mixed oxide route. Oxygen vacancies (VO••) played a major role in the semi-conduction mechanism in low p(O2) for all compositions. Impedance spectroscopy and thermo-power data confirmed KNN to be n-type in low p(O2) in contradiction to previous reports of p-type behaviour. The best piezoelectric properties were observed for air- rather than N2-sintered samples with d33 = 125 pC/N and kp = 0.38 obtained for K0.51Na0.49NbO3. |
| Keywords | p-Type, n-Type, Low p(O2), Oxygen vacancies, Seebeck coefficient |
| Remark |
https://doi.org/10.1016/j.jeurceramsoc.2018.03.013 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Inter-diffusion across a direct p-n heterojunction of Li-doped NiO and Al-doped ZnO
| Authors |
|
| Source |
Solid State Ionics
Volume: 320,
Pages: 215-220 Time of Publication: 2018 |
| Abstract | We herein report inter-diffusion across the interface between p-type Ni0.98Li0.02O and n-type Zn0.98Al0.02O for various applications including p-n-heterojunction diodes and oxide thermoelectrics. Diffusion couples were made of polished surfaces of ceramic samples pre-sintered at 1250 and 1350 °C for Ni0.98Li0.02O and Zn0.98Al0.02O, respectively. The inter-diffusion couples were annealed at 900–1200 °C for 160 h in ambient air. Electron Probe Micro Analysis (EPMA) was used to acquire diffusion profiles, followed by fitting to Ficks second law and Whipple–Le Claires models for bulk and grain-boundary diffusion calculation, respectively. Zn2+ diffused into Ni0.98Li0.02O mainly by bulk diffusion with an activation energy of 250 ± 10 kJ/mol, whereas Ni2+ diffused into Zn0.98Al0.02O by both bulk and enhanced grain boundary diffusion with activation energies of 320 ± 120 kJ/mol and 245 ± 50 kJ/mol, respectively. The amount of Al3+ diffused from the Al-doped ZnO into the NiO phase was too small for a corresponding diffusion coefficient to be calculated. Li-ion distribution and diffusivity were not determined due to lack of analyzer sensitivity for Li. The bulk and effective diffusivities of Zn2+ and Ni2+ into NiO and ZnO enable prediction of inter-diffusion lengths as a function of time and temperature, allowing estimates of device performance, stability, and lifetimes at different operation temperatures. |
| Keywords | NiO, ZnO, Cation diffusion, Grain-boundary diffusion, p-n junction |
| Remark |
https://doi.org/10.1016/j.ssi.2018.03.011 Ask ChatGPT for Link Ask ChatGPT to Summarize |
The Band Gap of BaPrO3 Studied by Optical and Electrical Methods
| Authors |
|
| Source |
Journal of the American Ceramic Society
Volume: 99,
Issue: 2,
Pages: 492–498 Time of Publication: 2016 |
| Abstract | We report on measurements of the electrical and optical properties of BaPrO3. The temperature dependences of the electrical conductivity σ and the Seebeck coefficient α of polycrystalline samples were studied over a wide temperature range (300°C–1050°C). At lower temperatures, the observed charge transport can be described as thermally activated hopping of electron-based small polarons with an activation energy of 0.37 eV. An observed change in temperature dependence of both σ and α around 700°C was observed and interpreted as a transition from extrinsic to intrinsic carrier transport. The intrinsic conduction can be modeled with an apparent electrical band gap of ~2 eV. Optical absorption and emission spectroscopy in the UV–VIS–NIR range revealed a series of characteristic absorption thresholds and the type of optical transitions was identified by combining transmittance and diffuse-reflectance spectroscopy methods. An absorption edge of indirect type with onset at 0.6 eV is attributed to small polaron effects. The higher lying absorption thresholds of direct origin positioned at around 1.8 and 3.8 eV are correlated with thermal activation parameters from electrical measurements and discussed in terms of the band gap of BaPrO3. |
| Remark |
DOI: 10.1111/jace.13961 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Enhanced Flexible Thermoelectric Generators Based on Oxide–Metal Composite Materials
| Authors |
|
| Source |
Journal of Electronic Materials
Volume: 46,
Issue: 4,
Pages: 2356–2365 Time of Publication: 2017 |
| Abstract | The thermoelectric performance of flexible thermoelectric generator stripes was investigated in terms of different material combinations. The thermoelectric generators were constructed using Cu-Ni-Mn alloy as n-type legs while varying the p-type leg material by including a metallic silver phase and an oxidic copper phase. For the synthesis of Ca3Co4O9/CuO/Ag ceramic-based composite materials, silver and the copper were added to the sol–gel batches in the form of nitrates. For both additional elements, the isothermal specific electronic conductivity increases with increasing amounts of Ag and CuO in the samples. The amounts for Ag and Cu were 0 mol.%, 2 mol.%, 5 mol.%, 10 mol.%, and 20 mol.%. The phases were confirmed by x-ray diffraction. Furthermore, secondary electron microscopy including energy dispersive x-ray spectroscopy were processed in the scanning electron microscope and the transmission electron microscope. For each p-type material, the data for the thermoelectric parameters, isothermal specific electronic conductivity σ and the Seebeck coefficient α, were determined. The p-type material with a content of 5 mol.% Ag and Cu exhibited a local maximum of the power factor and led to the generator with the highest electric power output Pel. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Microstructural design of CaMnO3 and its thermoelectric proprieties.
| Author |
|
| Source |
dissertation
Time of Publication: 2015
|
| Remark |
Norwegian University of Science and Technology, Department of Materials Science and Engineering Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Improvement of thermoelectric properties of lanthanum cobaltate by Sr and Mn co-substitution
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 735,
Pages: 1787–1791 Time of Publication: 2018 |
| Abstract | We report thermoelectric (TE) properties of Sr and Mn co-substituted LaCoO3 system from room temperature to 700 K. Sr-substitutions at La and Mn at Co site in LaCoO3 improves the electrical conductivity (σ). Thermal conductivity (κ) of all the samples increases with the increase in temperature but decreases with the substitution in LaCoO3. An estimation of the electronic thermal conductivity (κe) suggests a dominant phonon contribution to thermal conductivity in this system. A maximum value of the figure of merit is 0.14 at 480 K for La0.95Sr0.05Co0.95Mn0.05O3. |
| Keywords | Powders: solid-state reaction; Thermal conductivity; Electrical conductivity; Perovskites |
| Remark |
https://doi.org/10.1016/j.jallcom.2017.11.334 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Influence of processing on stability, microstructure and thermoelectric properties of Ca3Co4 − xO9 + δ
| Authors |
|
| Source |
Journal of the European Ceramic Society
Time of Publication: 2017
|
| Abstract | Due to high figure of merit, Ca3Co4 − xO9 + δ (CCO) has potential as p-type material for high-temperature thermoelectrics. Here, the influence of processing including solid state sintering, spark plasma sintering and post-calcination on stability, microstructure and thermoelectric properties is reported. By a new post-calcination approach, single-phase materials were obtained from precursors to final dense ceramics in one step. The highest zT of 0.11 was recorded at 800 °C for CCO with 98 and 72% relative densities. In situ high-temperature X-ray diffraction in air and oxygen revealed a higher stability of CCO in oxygen (∼970 °C) than in air (∼930 °C), with formation of Ca3Co2O6 which also showed high stability in oxygen, even at 1125 °C. Since achievement of phase pure high density CCO by post-calcination method in air is challenging, the phase stability of CCO in oxygen is important for understanding and further improvement of the method. |
| Keywords | Ca3Co4 − xO9 + δ, Post calcination, Phase stability, Microstructure, Thermoelectric performance |
| Remark |
Available online 6 November 2017, https://doi.org/10.1016/j.jeurceramsoc.2017.11.011 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Defect chemistry and electrical properties of BiFeO3
| Authors |
|
| Source |
Journal of Materials Chemistry C
Issue: 38
Time of Publication: 2017
|
| Abstract | BiFeO3 attracts considerable attention for its rich functional properties, including room temperature coexistence of magnetic order and ferroelectricity and more recently, the discovery of conduction pathways along ferroelectric domain walls. Here, insights into the defect chemistry and electrical properties of BiFeO3 are obtained by in situ measurements of electrical conductivity, σ, and Seebeck coefficient, α, of undoped, cation-stoichiometric BiFeO3 and acceptor-doped Bi1−xCaxFeO3−δ ceramics as a function of temperature and oxygen partial pressure pO2. Bi1−xCaxFeO3−δ exhibits p-type conduction; the dependencies of σ and α on pO2 show that Ca dopants are compensated mainly by oxygen vacancies. By contrast, undoped BiFeO3 shows a simultaneous increase of σ and α with increasing pO2, indicating intrinsic behavior with electrons and holes as the main defect species in almost equal concentrations. The pO2-dependency of σ and α cannot be described by a single point defect model but instead, is quantitatively described by a combination of intrinsic and acceptor-doped characteristics attributable to parallel conduction pathways through undoped grains and defect-containing domain walls; both contribute to the total charge transport in BiFeO3. Based on this model, we discuss the charge transport mechanism and carrier mobilities of BiFeO3 and show that several previous experimental findings can readily be explained within the proposed model. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Improvement of thermoelectric properties of lanthanum cobaltate by Sr and Mn co-substitution
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 735,
Pages: 1787–1791 Time of Publication: 2017-12 |
| Abstract | We report thermoelectric (TE) properties of Sr and Mn co-substituted LaCoO3 system from room temperature to 700 K. Sr-substitutions at La and Mn at Co site in LaCoO3 improves the electrical conductivity (σ). Thermal conductivity (κ) of all the samples increases with the increase in temperature but decreases with the substitution in LaCoO3. An estimation of the electronic thermal conductivity (κe) suggests a dominant phonon contribution to thermal conductivity in this system. A maximum value of the figure of merit is 0.14 at 480 K for La0.95Sr0.05Co0.95Mn0.05O3. |
| Keywords | Seebsys, Powders: solid-state reaction, Thermal conductivity, Electrical conductivity, Perovskites |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Fabrication and testing of unileg oxide thermoelectric device
| Authors |
|
| Source |
API Conference Proceedings
Time of Publication: 2017
|
| Abstract | A prototype of oxide thermoelectric unileg device was fabricated. This device was based on only n-legs made of La doped calcium manganate. The powder was synthesized, characterised and consolidated in rectangular thermoelements. A 3×3 device was fabricated by fitting 9 rectangular bars in alumina housing and connected by silver strips. The device has been tested under large temperature difference (ΔT=480°C) using an indegenous system. An open circuit voltage of 468 mV was obtained for a nine leg unileg device. The device exhibits a internal resistance of ∼1Ω. The maximum power output for this nine leg device reached upto 50 mW in these working condition |
| Keywords | Seebsys |
On the formation of phases and their influence on the thermal stability and thermoelectric properties of nanostructured zinc antimonide
| Authors |
|
| Source |
Journal of Physics D: Applied Physics
Volume: 50,
Issue: 1
Time of Publication: 2016-11
|
| Abstract | To investigate the thermal reliability of the structure and thermoelectric properties of the zinc antimony compounds, undoped (Zn4Sb3) and doped (Zn4Sb2.95Sn0.05 and Co0.05Zn3.95Sb3) zinc antimonide samples were processed using the powder metallurgy route. It was observed that the as-prepared undoped sample contains a pure β-Zn4Sb3 phase, whereas the doped samples consist of Ω-ZnSb as the major phase and β-Zn4Sb3 as the minor phase. Differential scanning calorimetry analysis confirms the stability of the β-Zn4Sb3 phase up to 600 K. X-ray diffraction data of the undoped and doped samples show that the nanocrystallinity of the as-prepared samples is retained after one thermal cycle. The thermal bandgap, thermopower and thermal conductivity are not affected by the thermal cycle for the doped samples. A maximum power factor of 0.6 mW m−1 K−2 was achieved in the Sn-doped sample (Zn4Sb2.95Sn0.05). This is enhanced to 0.72 mW m−1 K−2 after one thermal cycle at 650 K under Ar atmosphere and slightly decreases after the third thermal cycle. In the case of the Co-doped sample (Co0.05Zn3.95Sb3), the power factor increases from 0.4 mW m−1 K−2 to 0.7 mW m−1 K−2 after the third thermal cycle. A figure of merit of ~0.3 is achieved at 573 K in the Zn4Sb2.95Sn0.05 sample. The results from the nanoindentation experiment show that Youngs modulus of the Sn-doped sample (Zn4Sb2.95Sn0.05) after the thermal cycle is enhanced (96 GPa) compared to the as-prepared sample (~76 GPa). These important findings on the thermal stability of the thermoelectric and mechanical properties of Sn-doped samples (Zn4Sb2.95Sn0.05) confirm that Sn-doped zinc antimonide samples can be used as efficient thermoelectric materials for device applications. |
| Keywords | Seebsys |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
The effect of Cu2O nanoparticle dispersion on the thermoelectric properties of n-type skutterudites
| Authors |
|
| Source |
Journal of Physics D: Applied Physics
Volume: 48,
Issue: 45
Publisher: IOP Publishing Ltd,
Time of Publication: 2015-11
|
| Abstract | We report the thermoelectric properties of Ba0.4Co4Sb12 and Sn0.4Ba0.4Co4Sb12 skutterudites dispersed with Cu2O nanoparticles. The samples were synthesized by ball milling and consolidated by spark plasma sintering. Dispersion of Cu2O is found to significantly influence the electrical resistivity and thermopower at high temperatures with a more pronounced effect on the electrical resistivity due to the energy filtering effect at the interface between Cu2O nanoparticles and a Ba0.4Co4Sb12 and Sn0.4Ba0.4Co4Sb12 matrix. At 573 K, the electrical resistivity of Ba0.4Co4Sb12 decreases from 5.01 × 10−5 Ohmm to 2.98 × 10−5 Ohmm upon dispersion of Cu2O. The dispersion of Cu2O reduces the thermal conductivity of the samples from 300 K and above by increasing the phonon scattering. The lowest observed thermal conductivity at 573 K is found to be 2.001 W mK−1 in Cu2O dispersed Ba0.4Co4Sb12 while it is 2.91 W mK−1 in the Ba0.4Co4Sb12 sample without Cu2O dispersion. Hence Cu2O dispersion plays a significant role in the thermoelectric properties and a maximum figure of merit (ZT ) ~ 0.92 is achieved in Cu2O dispersed Ba0.4Co4Sb12 at 573 K which is more than 200% compared to the pure Ba0.4Co4Sb12 sample. The results from nanoindentation experiments show that the Cu2O dispersed sample (Cu2O + Sn0.4Ba0.4Co4Sb11.6) has a higher reduced Youngs modulus (~139 GPa) than the pure Sn0.4Ba0.4Co4Sb11.6 sample (~128 GPa). |
| Keywords | Seebsys |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Phase stability and thermoelectric properties of Cu10.5Zn1.5Sb4S13 tetrahedrite
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 667,
Pages: 323-328 Time of Publication: 2016-05 |
| Abstract | Cu10.5Zn1.5Sb4S13 tetrahedrite compound was prepared by mechanical milling of Cu2S, ZnS and Sb2S3 powders and spark plasma sintered (SPS) to dense samples. The phase formation, chemical homogeneity, thermal stability of the compound and the thermoelectric properties of the sintered samples were evaluated. Single phase tetrahedrite with the crystallite size of 40 nm was obtained after 30 h of milling followed by annealing at 573 K for 6 h in an argon atmosphere. In-situ high-temperature X-ray diffraction studies revealed that the phase is stable up to 773 K. The Seebeck coefficient of the sintered samples of density >98% shows p-type behavior with maximum thermopower of 170 μV/K at 573 K. The electrical resistivity (ρ) decreases with temperature up to 475 K and then increases. A low thermal conductivity of 0.5 W/(m⋅K), in combination with moderate power factor gave a maximum ZT of ∼0.038 at 573 K in Cu10.5Zn1.5Sb4S13 sample having a grain size of ∼200 nm. |
| Keywords | Seebsys, Thermoelectric, Tetrahedrite, Solid state reactions, Spark plasma sintering, Figure of merit |
| Remark |
Cu10.5Zn1.5Sb4S13 Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Relating defect chemistry and electronic transport in the double perovskite Ba1−xGd0.8La0.2+xCo2O6−δ (BGLC)
| Authors |
|
| Source |
Journal of Materials Chemistry A
Volume: 5,
Pages: 15743-15751 Time of Publication: 2017 |
| Abstract | Rare earth double perovskites comprise a class of functional oxides with interesting physiochemical properties both for low- and high-temperature applications. However, little can be found relating electrical properties with equilibrium thermodynamics of non-stoichiometry and defects. In the present work, a comprehensive and generally applicable defect chemical model is developed to form the link between the defect chemistry and electronic structure of partially substituted BGLC (Ba1−xGd0.8La0.2+xCo2O6−δ, 0 ≤ x ≤ 0.5). The equilibrium oxygen content of 4 different compositions is determined as a function of pO2 and temperature by thermogravimetric analysis, and combined with defect chemical modelling to obtain defect concentrations and thermodynamic parameters. Oxidation enthalpies determined by TG-DSC become increasingly exothermic (−50 to −120 kJ mol−1) with increased temperature and oxygen non-stoichiometry for all compositions, in excellent agreement with the thermodynamic parameters obtained from the defect chemical model. All compositions display high electrical conductivities (500 to 1000 S cm−1) with shallow pO2-dependencies and small and positive Seebeck coefficients (3 to 15 μV K−1), indicating high degree of degeneracy of the electronic charge carriers. The complex electrical properties of BGLC at elevated temperatures is rationalized by a two-band conduction model where highly mobile p-type charge carriers are transported within the valence band, whereas less mobile “n-type” charge carriers are located in narrow Co 3d band. |
| Remark |
DOI: 10.1039/C7TA02659E Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Porous Ca3Co4O9 with enhanced thermoelectric properties derived from Sol–Gel synthesis
| Authors |
|
| Source |
Journal of the European Ceramic Society
Time of Publication: 2017
|
| Abstract | Highly porous Ca3Co4O9 thermoelectric oxide ceramics for high-temperature application were fabricated by sol–gel synthesis and subsequent conventional sintering. Growth mechanism of misfit-layered Ca3Co4O9 phase, from sol–gel synthesis educts and upcoming intermediates, was characterized by in-situ X-ray diffraction, scanning electron microscopy and transmission electron microscopy investigations. The Ca3Co4O9 ceramic exhibits a relative density of 67.7%. Thermoelectric properties were measured from 373 K to 1073 K. At 1073 K a power factor of 2.46 μW cm−1 K−2, a very low heat conductivity of 0.63 W m−1 K−1 and entropy conductivity of 0.61 mW m−1 K−2 were achieved. The maintained figure of merit ZT of 0.4 from sol–gel synthesized Ca3Co4O9 is the highest obtained from conventional, non-doped Ca3Co4O9. The high porosity and consequently reduced thermal conductivity leads to a high ZT value. |
| Keywords | Thermoelectricity; Thermal conductivity; Porosity; Oxide; Ca3Co4O9 |
| Remark |
https://doi.org/10.1016/j.jeurceramsoc.2017.04.059 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Thermal stability and enhanced thermoelectric properties of the tetragonal tungsten bronzes Nb8−xW9+xO47 (0 < x < 5)
| Authors |
|
| Source |
Journal of Materials Chemistry A
Time of Publication: 2017
|
| Abstract | Thermoelectric materials are believed to play a fundamental role in the energy field over the next years thanks to their ability of directly converting heat into usable electric energy. To increase their integration in the commercial markets, improvements of the efficiencies are needed. At the same time, cheap and non-toxic materials are required along with easily upscalable production cycles. Compounds of the tetragonal tungsten bronze (TTB) series Nb8−xW9+xO47 fulfill all these requirements and are promising materials. Their adaptive structure ensures glass-like values of the thermal conductivity, and the substitution on the cation side allows a controlled manipulation of the electronic properties. In this contribution we report the stability study of the two highly substituted samples of the series, Nb5W12O47 (x = 3) and Nb4W13O47 (x = 4), when subjected to thermal cycling. Moreover, we show the results of the thermoelectric characterization of these samples. The two compounds have not been affected by the thermal treatment and showed an improvement of the thermoelectric performances up to a zT = 0.2 above 1000 K. |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Conduction Mechanisms in BaTiO3–Bi(Zn1/2Ti1/2)O3 Ceramics
| Authors |
|
| Source |
J. Am. Ceram. Soc.
Time of Publication: 2016
|
| Abstract | Polycrystalline BaTiO3–Bi(Zn1/2Ti1/2)O3 (BT–BZT) ceramics have superior dielectric properties for high-temperature and high-energy density applications as compared to the existing materials. While it has been shown that the addition of BZT to BT leads to an improvement in resistivity by two orders of magnitude, in this study impedance spectroscopy is used to demonstrate a novel change in conduction mechanism. While nominally undoped BT exhibits extrinsic-like p-type conduction, it is reported that BT–BZT ceramics exhibit intrinsic n-type conduction using atmosphere-dependent conductivity measurements. Annealing studies and Seebeck measurements were performed and confirmed this result. For BT, resistivity values were higher for samples annealed in nitrogen as compared to oxygen, whereas the opposite responses were observed for BZT-containing solid solutions. This suggests a fundamental change in the defect equilibrium conditions upon the addition of BZT to the solid solution that lowered the carrier concentration and changed the sign of the majority charge carrier. This is then also linked to the observed improvement in resistivity in BT–BZT ceramics as compared to undoped BT. |
| Remark |
doi:10.1111/jace.14313 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Phase stability and thermoelectric properties of Cu10.5Zn1.5Sb4S13 tetrahedrite
| Authors |
|
| Source |
Journal of Alloys and Compounds
Volume: 667,
Pages: 323–328 Time of Publication: 2016 |
| Abstract | Cu10.5Zn1.5Sb4S13 tetrahedrite compound was prepared by mechanical milling of Cu2S, ZnS and Sb2S3 powders and spark plasma sintered (SPS) to dense samples. The phase formation, chemical homogeneity, thermal stability of the compound and the thermoelectric properties of the sintered samples were evaluated. Single phase tetrahedrite with the crystallite size of 40 nm was obtained after 30 h of milling followed by annealing at 573 K for 6 h in an argon atmosphere. In-situ high-temperature X-ray diffraction studies revealed that the phase is stable up to 773 K. The Seebeck coefficient of the sintered samples of density >98% shows p-type behavior with maximum thermopower of 170 μV/K at 573 K. The electrical resistivity (ρ) decreases with temperature up to 475 K and then increases. A low thermal conductivity of 0.5 W/(m⋅K), in combination with moderate power factor gave a maximum ZT of ∼0.038 at 573 K in Cu10.5Zn1.5Sb4S13 sample having a grain size of ∼200 nm. |
| Keywords | Thermoelectric; Tetrahedrite; Solid state reactions; Spark plasma sintering; Figure of merit |
| Remark |
doi:10.1016/j.jallcom.2016.01.094 Ask ChatGPT for Link Ask ChatGPT to Summarize |
The effect of Cu2O nanoparticle dispersion on the thermoelectric properties of n-type skutterudites
| Authors |
|
| Source |
Journal of Physics D: Applied Physics
Volume: 48,
Issue: 45
Time of Publication: 2015
|
| Abstract | We report the thermoelectric properties of Ba0.4Co4Sb12 and Sn0.4Ba0.4Co4Sb12 skutterudites dispersed with Cu2O nanoparticles. The samples were synthesized by ball milling and consolidated by spark plasma sintering. Dispersion of Cu2O is found to significantly influence the electrical resistivity and thermopower at high temperatures with a more pronounced effect on the electrical resistivity due to the energy filtering effect at the interface between Cu2O nanoparticles and a Ba0.4Co4Sb12 and Sn0.4Ba0.4Co4Sb12 matrix. At 573 K, the electrical resistivity of Ba0.4Co4Sb12 decreases from 5.01 × 10−5 Ωm to 2.98 × 10−5 Ωm upon dispersion of Cu2O. The dispersion of Cu2O reduces the thermal conductivity of the samples from 300 K and above by increasing the phonon scattering. The lowest observed thermal conductivity at 573 K is found to be 2.001 W mK−1 in Cu2O dispersed Ba0.4Co4Sb12 while it is 2.91 W mK−1 in the Ba0.4Co4Sb12 sample without Cu2O dispersion. Hence Cu2O dispersion plays a significant role in the thermoelectric properties and a maximum figure of merit (ZT ) ~ 0.92 is achieved in Cu2O dispersed Ba0.4Co4Sb12 at 573 K which is more than 200% compared to the pure Ba0.4Co4Sb12 sample. The results from nanoindentation experiments show that the Cu2O dispersed sample (Cu2O + Sn0.4Ba0.4Co4Sb11.6) has a higher reduced Youngs modulus (~139 GPa) than the pure Sn0.4Ba0.4Co4Sb11.6 sample (~128 GPa). |
| Remark |
Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Tetragonal tungsten bronzes Nb8−xW9+xO47−δ: optimization strategies and transport properties of a new n-type thermoelectric oxide
| Authors |
|
| Source |
Materials Horizons
Issue: 5,
Pages: 519-527 Time of Publication: 2015 |
| Abstract | Engineering of nanoscaled structures may help controlling the electrical and thermal transport in solids, in particular for thermoelectric applications that require the combination of low thermal conductivity and low electrical resistivity. The tetragonal tungsten bronzes Nb8−xW9+xO47 (TTB) allow a continuous variation of the charge carrier concentration while fulfilling at the same time the concept of a “phonon-glass electron-crystal” through a layered nanostructure defined by intrinsic crystallographic shear planes. The thermoelectric properties of the tetragonal tungsten bronzes Nb8−xW9+xO47−δ (0 < x < 2) were studied in the temperature range from 373 to 973 K. Structural defects and the thermal stability under various oxygen partial pressure pO2 were investigated by means of thermogravimetry, HR-TEM, and XRD. Nb8W9O47−δ was found stable at 973 K and a pO2 of ≈10−15 atm. The oxygen nonstoichiometry δ can reach up to 0.3, depending on the applied atmosphere. By increasing the substitution level x, the electrical resistivity ρ and the Seebeck coefficient S decreased. For x = 2, ρ reached 20 mΩ cm at 973 K, combined with a Seebeck coefficient of approximately −120 μV K−1. The thermal conductivity was low for all samples, ranging from 1.6 to 2.0 W K−1 m−1, attributed to the complex crystal structure. The best thermoelectric figure of merit zT of the investigated samples was 0.043, obtained for x = 2 at 973 K, but it is expected to increase significantly upon a further increase of x. The control of the oxygen non-stoichiometry δ opens a second independent optimization strategy for tetragonal tungsten bronzes. |
| Remark |
DOI: 10.1039/C5MH00033E Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Electrical conductivity and thermopower of (1 − x) BiFeO3 – xBi0.5K0.5TiO3 (x = 0.1, 0.2) ceramics near the ferroelectric to paraelectric phase transition
| Authors |
|
| Source |
Physical Chemistry Chemical Physics
Volume: 17,
Issue: 14,
Pages: 9420-9428 Time of Publication: 2015 |
| Abstract | Ferroelectric BiFeO3 has attractive properties such as high strain and polarization, but a wide range of applications of bulk BiFeO3 are hindered due to high leakage currents and a high coercive electric field. Here, we report on the thermal behaviour of the electrical conductivity and thermopower of BiFeO3 substituted with 10 and 20 mol% Bi0.5K0.5TiO3. A change from p-type to n-type conductivity in these semi-conducting materials was demonstrated by the change in the sign of the Seebeck coefficient and the change in the slope of the isothermal conductivity versus partial pressure of O. A minimum in the isothermal conductivity was observed at [similar]10−2 bar O2 partial pressure for both solid solutions. The strong dependence of the conductivity on the partial pressure of O2 was rationalized by a point defect model describing qualitatively the conductivity involving oxidation/reduction of Fe3+, the dominating oxidation state of Fe in stoichiometric BiFeO3. The ferroelectric to paraelectric phase transition of 80 and 90 mol% BiFeO3 was observed at 648 ± 15 and 723 ± 15 °C respectively by differential thermal analysis and confirmed by dielectric spectroscopy and high temperature powder X-ray diffraction. |
| Remark |
DOI: 10.1039/C5CP00266D Original Link (may be broken) Ask ChatGPT for Link Ask ChatGPT to Summarize |
Versatile apparatus for thermoelectric characterization of oxides at high temperatures
| Authors |
|
| Source |
Review of Scientific Instruments
Volume: 85,
Pages: 103906 Time of Publication: 2014 |
| Abstract | An apparatus for measuring the Seebeck coefficient and electrical conductivity is presented and characterized. The device can be used in a wide temperature range from room temperature to 1050 °C and in all common atmospheres, including oxidizing, reducing, humid, and inert. The apparatus is suitable for samples with different geometries (disk-, bar-shaped), allowing a complete thermoelectric characterization (including thermal conductivity) on a single sample. The Seebeck coefficient α can be measured in both sample directions (in-plane and cross-plane) simultaneously. Electrical conductivity is measured via the van der Pauw method. Perovskite-type CaMnO3 and the misfit cobalt oxide (Ca2CoO3) q (CoO2) are studied to demonstrate the temperature range and to investigate the variation of the electrical properties as a function of the measurement atmosphere. |
| Remark |
http://dx.doi.org/10.1063/1.4897489 Ask ChatGPT for Link Ask ChatGPT to Summarize |
Thermoelectric Properties of A-site Deficient Lanthanum Substituted Strontium Titanate
| Author |
|
| Source |
Time of Publication: 2014
|
Multilayered thin films for oxidation protection of Mg2Si thermoelectric material at middle–high temperatures
| Authors |
|
| Source |
Thin Solid Films
Volume: 526,
Pages: 150–154 Time of Publication: 2012-12 |
| Abstract | Multilayered molybdenum silicide-based thin films were deposited via radio frequency magnetron sputtering in order to obtain efficient barrier against oxidation process which affected Mg2Si thermoelectric materials at middle–high temperatures. X ray diffraction, energy dispersive spectroscopy, secondary ion mass spectroscopy, field emission scanning electron microscopy (FE-SEM) and electrical measurements at high temperature were carried out in order to obtain, respectively, the structural, compositional, morphological and electrical characterization of coatings. Furthermore, the mechanical behavior of the thin film/Mg2Si-pellet system was observed in situ as a function of temperature by FE-SEM employing a heating module. Moreover, the barrier properties for oxygen protection after thermal treatment in air at high temperature were qualitatively evaluated. |
| Keywords | Thin film; Thermoelectric material; Magnesium silicide; Molybdenum silicide; Middle–high temperature |
Temperature dependent thermoelectric material power factor measurement system
| Authors |
|
| Source |
Review of scientific instruments
Volume: 81,
Issue: 075107
Publisher: American Institute of Physics,
Time of Publication: 2010-06
|
| Abstract | Thermoelectric materials can be used for cooling/heating applications, or converting waste heat into electricity. Novel thermoelectric materials have been discovered in recent years. Characterization of an electrical conductivity and thermopower of a sample from room temperature to ≥ 900 K is often necessary for thermoelectric materials. This paper describes a system built for measurement of the power factor of thermoelectric materials from 300 to 1273 K. Characterization results of the system are also presented. |
Comperative Seebeck Coefficient Measurements on Ceramic and Compacted Powder Column Samples; Case of ZnO
| Authors |
|
| Source |
Proceeding of the 1st Nordic School and Symposium on Functional Energy Related Materials
NorFERM-2008, 3-7 October 2008, Gol, Norway Volume: R, Pages: 36 Time of Publication: 2008 |
Thermoelectric power of the mixed ionic-electronic conductor SrTi0.8Fe0.2O3-d in various atmospheres
| Authors |
|
| Source |
proc. Nordic Energy Research Workshop on "Hydrogen in Electrochemical Energy Conversion", Geilo
Volume: March 1999
Time of Publication: 1999
|
| thermoelectric | |
| Editors |
|
| Remark |
Bar sample, thermoelectric power (Seebeck coefficient) measurements Ask ChatGPT for Link Ask ChatGPT to Summarize |